Spinal cord stimulation
Spinal cord stimulation (SCS) is a treatment for chronic severe neuropathic pain. Electrodes are implanted into the spine, just behind the spinal cord. They stimulate the back of the spinal cord where the nerve fibres that carry sensation run upwards towards the head. Stimulating these fibres aims to mask painful signals with other less unpleasant sensations, often described as being like a buzzing feeling.
What is a spinal cord stimulation system made up of?
The spinal cord stimulation system consists of two components:
- an electrode that is implanted next to the spinal cord
- an implantable pulse generator (IPG) which is a small box that contains a battery and circuitry to produce the stimulus current
The electrode
This is made of soft silicone containing several metal contacts that deliver the electrical pulses. The electrodes come in a variety of sizes and shapes, some with more contacts than others. We choose the best type for each patient and this depends on things like the exact location of the pain and how widespread it is (e.g. does it affect only one leg or both?).
 A paddle electrode for spinal cord stimulation *
A paddle electrode for spinal cord stimulation *Some electrodes are shaped like a straight wire and these are intended to be passed into the spine through a large hollow needle. Other electrodes are shaped like a paddle, i.e. they have a wider and flatter end. These need a slightly bigger procedure to put them in, involving a small incision in the back, but they have more electrical contacts over a greater area of the spinal cord which allows greater flexibility in terms of stimulation patterns. The other advantage of paddle electrodes is that they rarely displace away from their intended position, a problem that is quite common with electrodes that are put in through a needle. For these reasons at OFN in general we use paddle electrodes and the discussion below refers to these.
The implanted pulse generator (IPG)
This is commonly referred to as the 'battery' but it also contains the circuitry that produces the small electrical current that is sent to the contacts on the electrode. If the electrode is being put in the lower back (e.g. for leg pain) the IPG is usually implanted under the skin of the abdomen (tummy). If the electrode is being put in the spine in the neck (e.g. for arm pain) the IPG may be implanted under the skin below the collar bone.
Two types of IPG are available:
- Rechargeable
- Non-rechargeable
For a description of the pros and cons of each type and how we choose between them, see our page on IPG types. For most patients we recommend a rechargeable IPG.
How is a spinal cord stimulator implanted?
Spinal cord stimulation is normally performed in two stages, both under general anaesthesia.
Stage 1
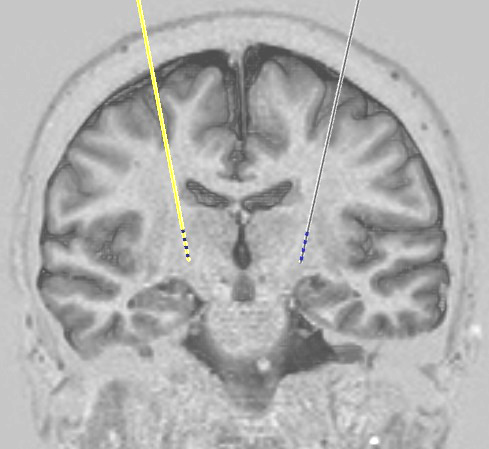
In stage 1 the electrode is inserted next to the spinal cord through a small incision (approximately one and a half inches long) in the midline of the back. Temporary extension leads are attached to the wires coming from the electrode and these are brought out through tiny holes in the skin nearby. Xrays are used during the procedure to ensure that the electrode is well positioned.
Testing
Over the next few days the stimulation is tested on the ward to see if it is effective. If the results are good we proceed to stage 2 but in the minority of cases where there is no beneficial effect we will simply remove the electrode.
Stage 2
The temporary extension leads are removed and discarded. Then the implantable pulse generator (battery) is implanted under the skin and the wires from the electrode are passed under the skin to connect to it. We then run electrical tests on the system to make sure all the parts and connections are working properly.
After surgery
It is usually possible to go home the day after stage 2. Instructions are provided for removal of stitches which can be done at the local GP practice.
Any problems
If there are any problems with a system that we have implanted, please contact us. Patients are always given the contact details of one of our specialist nurses who should be the first port of call. They are available every weekday during working hours to talk to patients, relatives, or their general practitioners. In the event of an emergency occurring out of hours, advice should be sought from the on-call neurosurgical service at the John Radcliffe Hospital in Oxford.
The commonest complication with neuromodulation systems is infection which occurs in around 1 case in 20. The most likely time for this to appear is within the first few weeks to months after implantation of a new system or replacement of an old battery or pump. If there are any concerns about the appearance of a wound or any other symptom that might suggest infection, CALL US. If you are a patient then for this specific issue please call us rather than your GP. If you are a GP then please do not attempt to treat a possible wound infection with antibiotics but call us instead.
* Image courtesy of Medtronic
Copyright OFN Wednesday, 11 February, 2015.